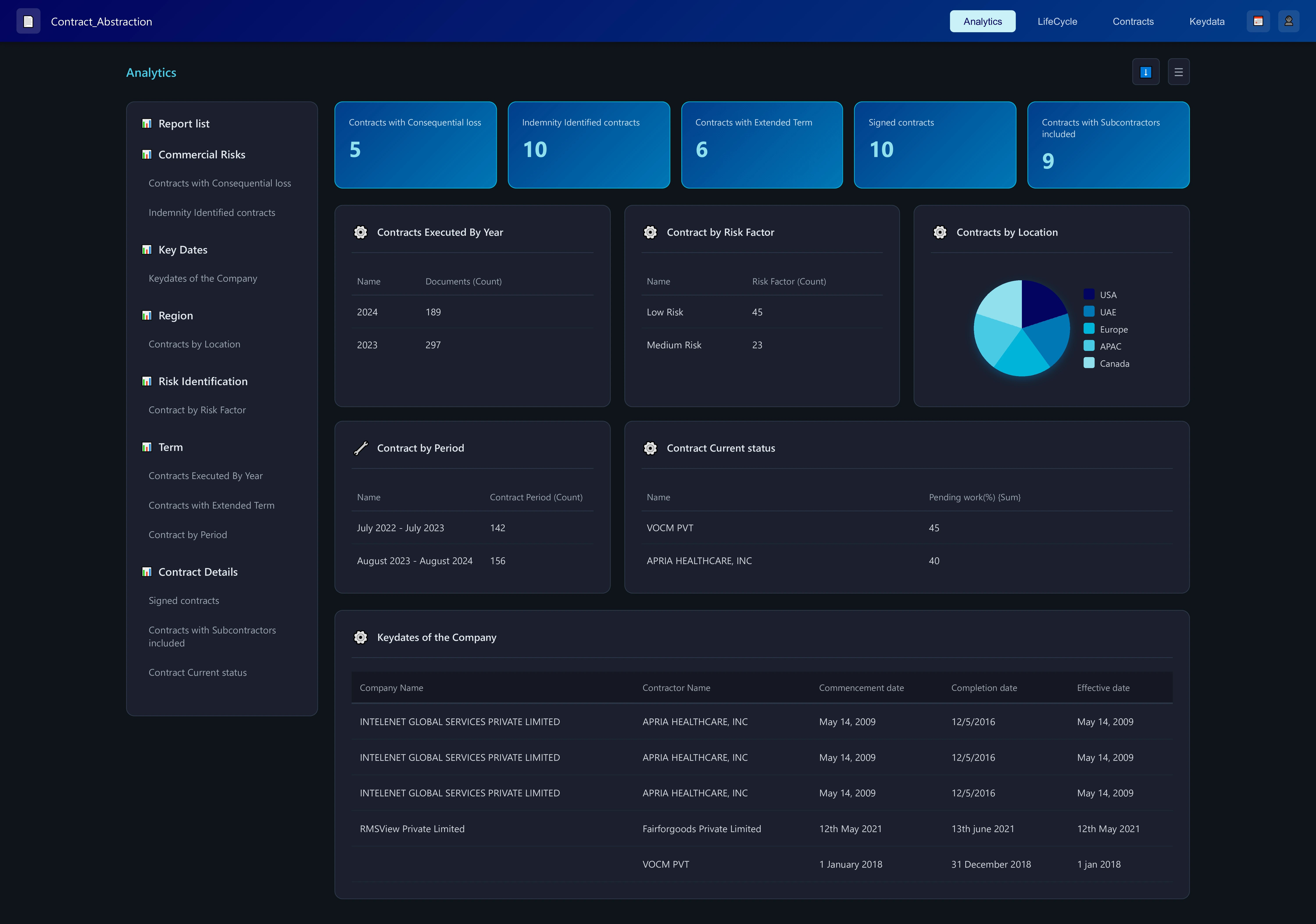
Process Investigation Workbench
Enable structured root-cause analysis and deviation tracking for process reliability and regulatory compliance.
Investigate, Document, and Resolve with Full Process Traceability
Capture data, deviations, and supporting records from batch operations. Correlate findings, identify root causes, and maintain an audit-ready history of every investigation cycle.
Improved Process Efficiency
Reduction in Processing Time
Increase in SOPs Processed
Data Extraction Accuracy
What Defines Us
Process Investigation Workbench brings structure and governance to deviation management in pharmaceutical manufacturing. It centralizes data from lab systems, manufacturing records, and quality logs—allowing investigators to analyze deviations using contextual data rather than isolated events. Each deviation, cause, and corrective action is tied to its source record, ensuring traceability through the entire batch lifecycle. The system supports cross-functional review by MSAT, QA, and production teams, with configurable workflows that align to GMP and regulatory requirements. Over time, aggregated data reveals recurring patterns, supporting process optimization and knowledge capture.
See Process Investigation Workbench in Action
View how deviations are logged, causes identified, and actions tracked—connecting every finding to its source data, document, or record in real time.
Configure with Ease
Set up AI capabilities quickly using intuitive, low-code tools.
Launch with Confidence
Deploy AI systems seamlessly and scale them efficiently.
Monitor & Optimize in Real-Time
Track performance metrics and continuously refine operations.
Evolve Continuously
Integrate feedback loops to adapt and improve your solutions.